Hypothermic Machine Preservation for Liver Transplantation
LifePort Liver Transporter design, engineering, and performance capabilities are based upon the LifePort prototype system used in the Columbia University Medical Center / New York Presbyterian Hospital and the proven technology platform of the market leading LifePort Kidney Transporter.
LifePort Liver Transporter is in the process of securing US and European regulatory registrations, and is not yet approved for commercial use.
Accessories & Support
LifePort Liver Transporter: Transforming Preservation
Over 2,300 patients in the United States were removed from the liver transplant waiting list in 2021, either because they died waiting for a donor liver or were deemed too sick to be transplanted.1 Increasing the number of organs available for transplantation, while reducing post-transplantation complications and shortening the length of hospital stay, are important clinical goals to improve patient outcomes.
In this video, learn how LifePort Liver Transporter is working to transform preservation of donor livers.
1. OPTN National data: Liver candidates removed from the Waiting List. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed October 2022.
Predicate Device and Preliminary Studies
A controlled clinical feasibility study at Columbia University Medical Center/New York Presbyterian Hospital using a prototype LifePort Liver Transporter system found 50 percent fewer patients had biliary complications with LifePort-perfused livers. Early allograft dysfunction was seen in 25 percent of static cold stored livers compared with 5 percent of LifePort-perfused livers (p = 0.08).2 Additionally, patients receiving LifePort perfused livers had a significantly lower length of hospital stay than patients with static cold stored livers (10.9 ± 4.7 days vs. 15.3 ± 4.9 days; p = 0.006).
A second controlled study investigated hypothermic machine preservation versus static cold storage of expanded criteria livers rejected by the originating UNOS region.3 During 12-months post transplantation there were significantly fewer biliary complications in the perfused group (4 vs. 13, respectively; p = 0.016), and early allograft dysfunction rates were also lower (19% vs. 30%). Mean hospital stay was also significantly shorter in the perfused group (13.6 vs. 21.1 days). The results suggest that hypothermic machine perfusion (HMP) of livers is safe and allows for transplantation of expanded criteria livers deemed untransplantable by multiple centers. Study investigators concluded that HMP has the potential to increase the donor pool and the availability of livers for patients on the waiting list.
Full Summary of the Clinical Experience for LifePort Liver Transporter
2. Guarrera JV, et al. Am J Transpl 2010;10:372–81.
3. Guarrera JV, et al. Am J Transpl 2015;15(1):161–9
Key Features of the LifePort Liver Transporter

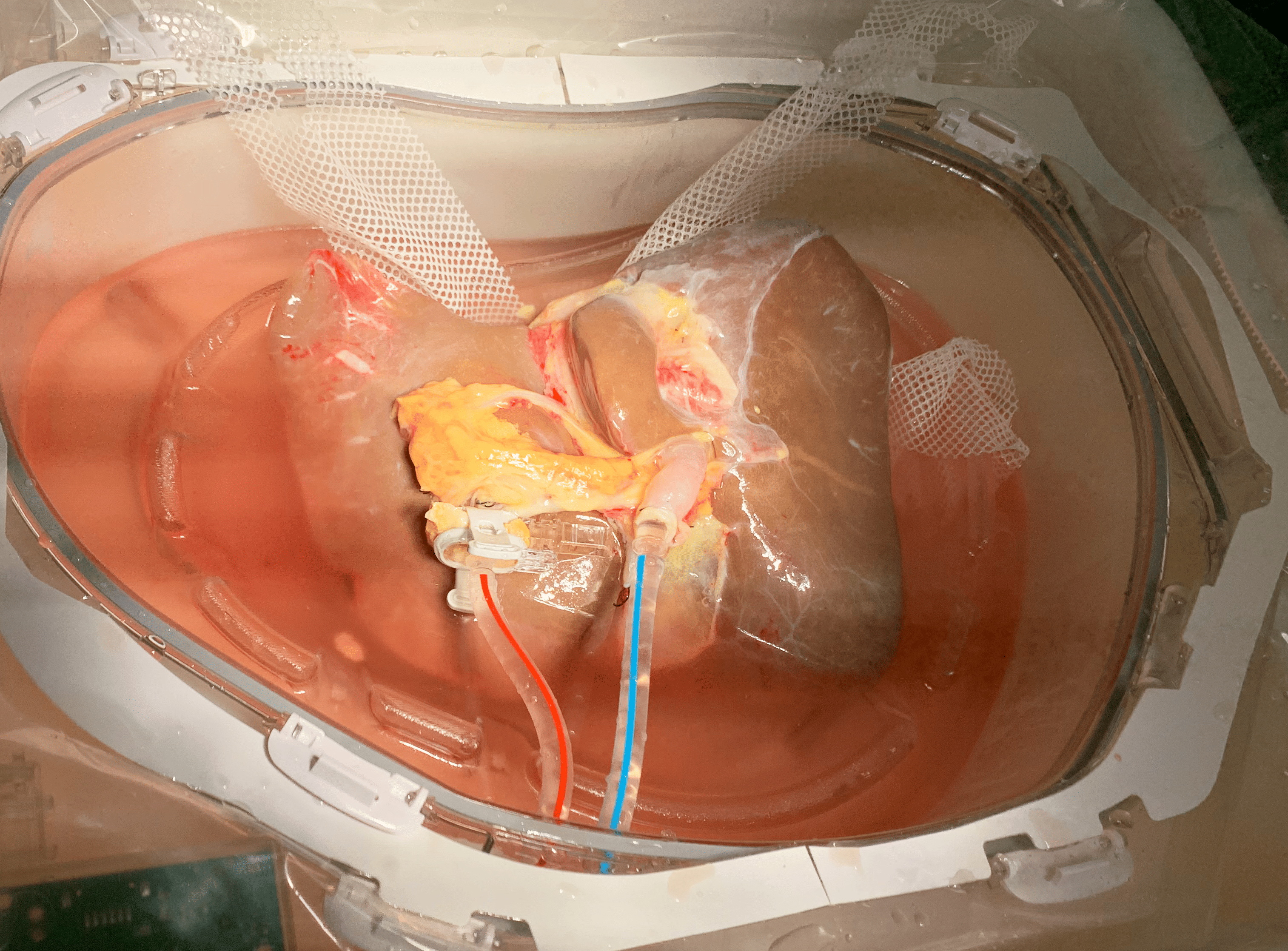
Dual Perfusion
LifePort Liver Transporter is designed to deliver precision-controlled perfusion of the hepatic artery and portal vein.

Programmable Display
The interface displays key data that track real-time organ perfusion status, organ ID number and blood type, cross-clamp and total infusion time, perfusate temperature, hepatic and portal flow, pressure, and total flow.

Trusted Technology
Hypothermic perfusion is supported by redundant preservation systems for safety – dynamic perfusion plus static cold storage. LifePort medical technology has been used by transplant surgeons in over 200,000 clinical procedures worldwide.
